Estimating Algae
Chlorophyll Analysis
Chlorophyll may be one of the most important molecules on earth. This combination of carbon, hydrogen, oxygen, nitrogen, and magnesium is capable of taking light-energy from the sun and turning it into sugars, a form of stored energy that the cells of the plant can use. Organisms that cannot photosynthesize ultimately need the energy produced by photosynthesis to survive.
Not only is chlorophyll responsible for the energy that drives life on earth, it is also the source of another requirement for life: oxygen. During photosynthesis oxygen is produced as a waste product and released into the water or air. This is important because oxygen is chemically reactive, readily bonding with other atoms and molecules. Without the continuous re-supplying associated with photosynthesis, our atmosphere would run out of the free, un-bound oxygen that we need to live.
Chlorophyll is actually a group of closely related pigments, differentiated as chlorophyll-a, -b, -c, -d, and -f. The chlorophyll pigments differ slightly in molecular structure and in the wavelength of light energy they absorb. Chlorophyll-a is the dominant pigment, existing in all algae, while the other forms of chlorophyll are not universal and are considered accessory pigments. Not all species of algae contain other forms of chlorophyll and the various taxonomic groups have different accessory pigments; for example the blue-green algae may contain chlorophyll-d and -f, while diatoms may only have chlorophyll-c as an accessory pigment. The presence of accessory pigments increases the range of light energy that can be used for photosynthesis.
Ruth Ann measures chlorophyll-a concentrations in the MU Limnology/Aquatic Ecology Laboratory in Columbia.
Ultimately, the LMVP is interested in the amount of algae growing in Missouri's lakes. One might wonder why the program focuses on chlorophyll pigment. The answer is simple; chlorophyll analysis is much easier, quicker, and cheaper than actually counting and measuring the algal cells in a water sample. Studies have shown that the relation between chlorophyll content and algal biomass is strong, and therefore chlorophyll concentration is used as an estimate of algal biomass across the planet.
The method used by the lab to analyze chlorophyll involves placing the filters processed by volunteers into a test tube with ethanol and then heating the tubes in a hot water bath. This process breaks the algal cells open, releasing the chlorophyll pigment into the ethanol. The ethanol is then analyzed in a fluorometer to determine chlorophyll concentrations. The fluorometer shines 430 nanometers (nm) light into the sample, 'exciting' the chlorophyll pigment, which in turn emits a quantity of 663 nm light. The fluorometer measures the intensity of emitted 663 nm light to quantify the chlorophyll concentration. See the Fall 2003 Water Line article for more information (http://www.lmvp.org/Waterline/fall2003/chlorophyll.htm)
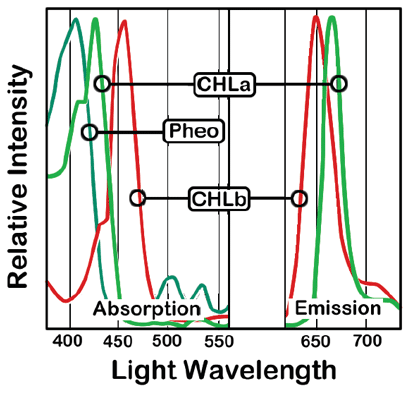
The wavelengths used in this method focus on detecting chlorophyll-a because it is the dominant chlorophyll pigment. Chlorophyll-b can interfere with the analysis because it gives off some 663 nm light when excited with 430 nm light. Thus the presence of this accessory pigment will lead to an overestimate of chlorophyll-a. Studies have shown that, at most, presence of chlorophyll-b could lead to a 10% overestimate of chlorophyll-a. This worst-case scenario involves a monoculture of a species that produces chlorophyll-b. Given that Missouri lakes tend to have a mix of algal species present, the amount of error associated with the presence of chlorophyll-b is much lower than 10%.
As chlorophyll degrades, parts of the molecule break off. When the magnesium atoms at chlorophyll's center are lost, it becomes pheophytin. Pheophytin-a and -b (breakdown products of chlorophyll-a and -b, respectively) also emit light at 663 nm when excited with light at 430 nm. A sample with high levels of pheophytin can lead to an overestimate of chlorophyll-a.
Using a fluorometer, chlorophyll is “excited” by light at 430 nanometers (nm). The intensity of light emitted by the chlorophyll molecules at 663 nm is measured and recorded.
Chlorophyll-b and pheophytin absorb and emit light at wavelengths that sometimes overlap with chlorophyll-a
The methodology used by the LMVP requires lab technicians to make two measurements on a given sample. The first measure is what we refer to as total chlorophyll. This terminology can cause some confusion, because we are not really measuring all the different chlorophylls (-a through -f), but instead are measuring chlorophyll-a with some potential for interference from chlorophyll-b, and the break-down pigments pheophytin-a and -b. After completing the initial measurement, lab techs add a small amount of acid to the remainder of the sample. This acidification strips the magnesium atoms from the chlorophyll, converting it into pheophytin. A second measure is taken, this time only measuring pheophytin. By plugging these two values into a formula we can calculate how much of the initial measurement was due to chlorophyll and how much was pheophytin. Thus we generate three values: Total Chlorophyll (or uncorrected chlorophyll), chlorophyll-a (corrected by accounting for the pheophytin), and pheophytin.
In most LMVP samples the amount of pheophytin break-down pigment is negligible. Samples collected in 2013 averaged 3.5µg/L pheophytin, which means the difference between our total or uncorrected chlorophyll and our chlorophyll-a values is small, averaging 1.4µg/L for 2013 season.
As with all environmental data, LMVP data are actually estimates. No measurements are perfect and we have to accept some level of error. Any interference due to the presence of chlorophyll-b pigment is minor relative to the other sources of variation in estimating chlorophyll concentrations in Missouri lakes. The methodology used by the MU Limnology Laboratory to generate chlorophyll data is precise in terms of its ability to measure low concentrations. Quality assurance and controls are implemented to assure the highest quality data.